016_alchemy research
b. 2020Experiments based on Fixation:
Fixation in the dictionary noun
1. the act of fixing or the state of being fixed.
2. Chemistry.
reduction from a volatile or fluid to a stable or solid form.
the process of converting atmospheric nitrogen into a useful compound, as a nitrate fertilizer.
3. Photography. the process of rendering an image permanent by removal of light-sensitive silver halides.
4. Psychoanalysis. a partial arrest of emotional and instinctual development at an early point in life, due to a severe traumatic experience or an overwhelming gratification.
5. a preoccupation with one subject, issue, etc.; obsession:
All her life she had a fixation on stories of violent death.
Fixation in alchemy
In alchemy, fixation is a process by which a previously volatile substance is "transformed" into a form (often solid) that is not affected by the fire. It separates the substance or object and puts it back in the same or different shape at a subatomic level. Fixation is sometimes listed as one of the processes required for the transformation of a substance, or completion of the alchemical magnum opus.
Experiment 1+2: Milk paint (pistachio color) + Earth pigments (Colonial Burnt Umber color)
-
Milk paint is a nontoxic water-based paint. It can be made from milk and lime, generally with pigments added for color.
- Earth pigments are naturally occurring minerals containing metal oxides, principally iron oxides and manganese oxides, that have been used since prehistoric times as pigments. The primary types are ochre, sienna and umber.


Milk Paint
Earth Pigments
Attempt NO.1:
Try to combine paints commonly used in woodworking with molten glass.
Poured the milk paint powder on the surface, then poured the glass over the milk paint. Poured the earth pigments over the glass, then cover with another layer of glass.
These powders are not very intended to be combined with glass. After taking it out of kiln, the milk paint that is not wrapped in the glass is still in powder condition that can be wiped off.





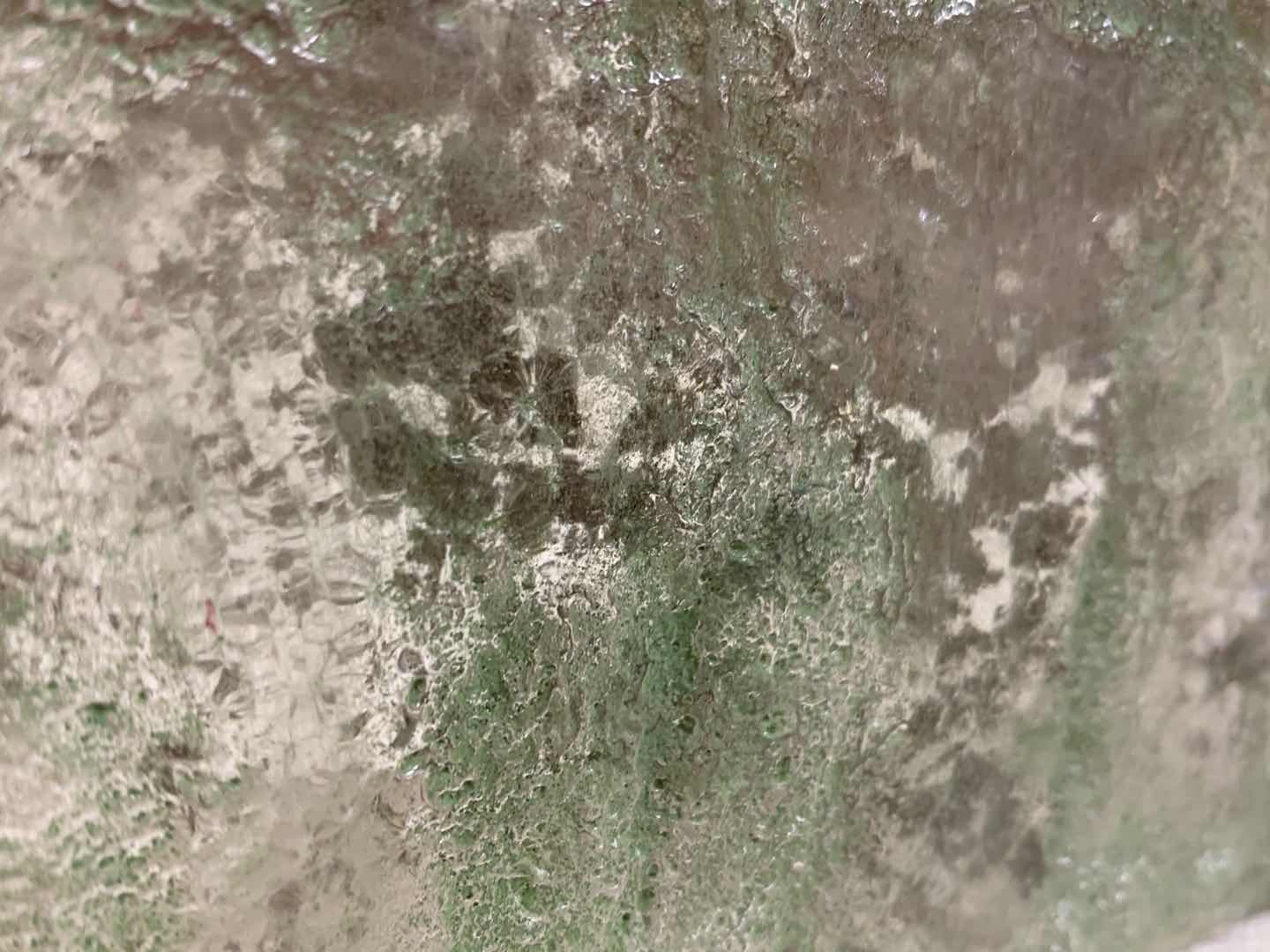


Experiment 3: Bone ash
- Bone ash is a white material produced by the calcination of bones. Typical bone ash consists of about 55.82% calcium oxide, 42.39% phosphorus pentoxide, and 1.79% water. The exact composition of these compounds varies depending upon the type of bones being used, but generally the formula for bone ash is: Ca5(OH)(PO4)3. Bone ash usually has a density around 3.10 g/mL and a melting point of 1670 °C (3038 °F). Most bones retain their cellular structure through calcination.
Attempt NO.1:
Try to capture the moment of dust flying in the molten glass. At the same time, hoped that the glass can be attached with a frosted effect.
Poured glass into a pentagon mold and stirred. Pressed with a semicircular mold.
After the glass was cooled, the texture of the ashes wrapped in it did not change, and the vortex shape formed by the stirring also achieved the purpose I wanted. However, due to the stirring action, a large number of small bubbles were mixed in the glass at the same time.







Attempt NO.2:
Poured and stirred into the ladle. Then poured the mixture of glass and bone ash onto the slab. Pressed with a semicircular mold to make it into a slab.
There are a lot of black particles in the cooled glass. It is not sure where it came from. It may be that the stirring stick contains impurities.




Experiment 4: Swarovski Crystals
- Swarovski glass is produced by melting a mixture of quartz sand, soda, potash and other ingredients at high temperatures.

Attempt NO.1:
Hoped that the molten glass can melt part of the crystal with heat, creating a watercolor effect.
Sandwiches a layer of crystal in between two layers of glass.
The thinner and smaller the crystal's volume is, the more successful the melting effect is obtained.


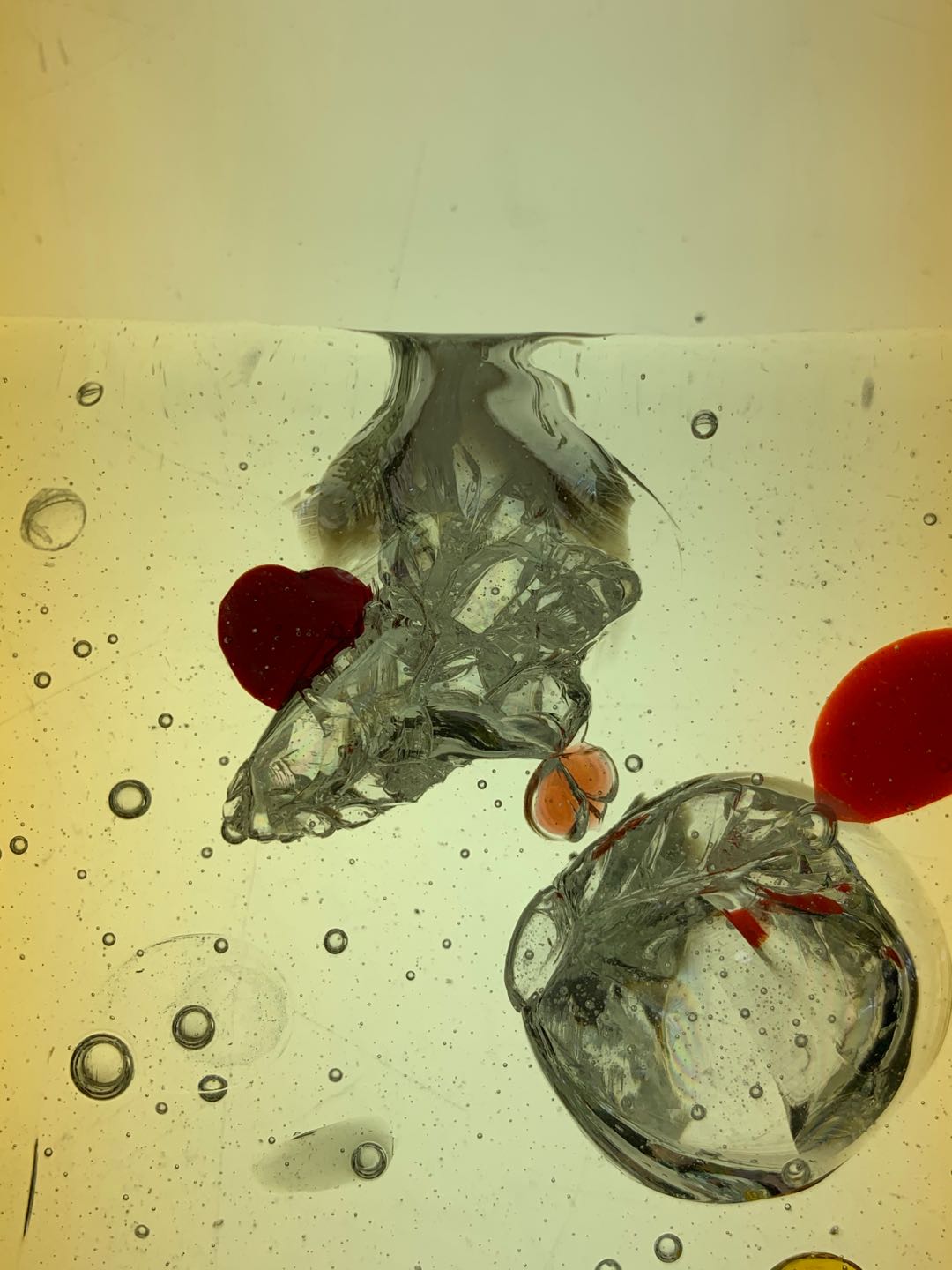

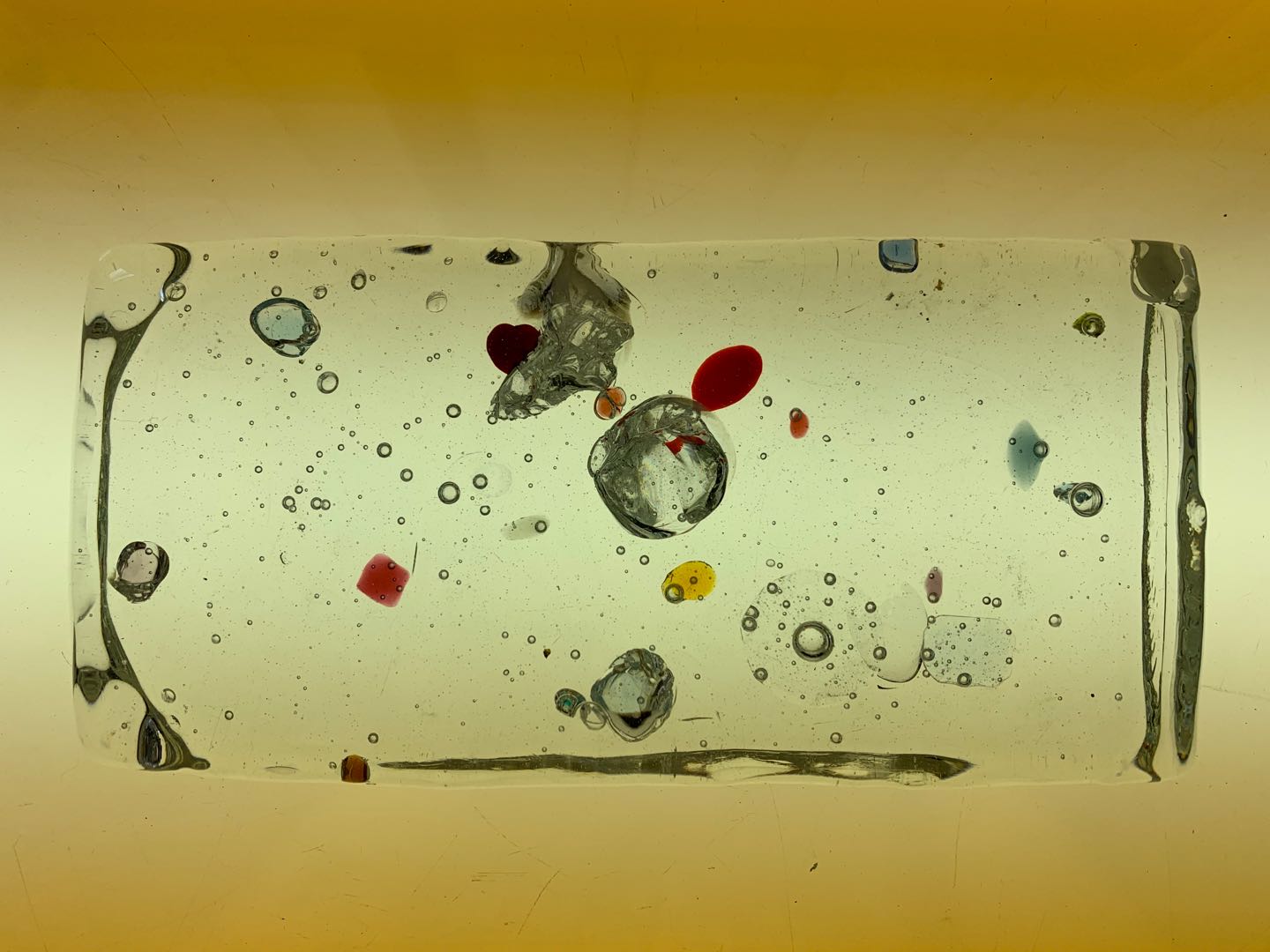

Attempt NO.2:
Hoped that the shape of the mold can be used to create a structure for the cooled glass.
Poured the molten glass on the top of the cylinder shape mold, then sprinkled the crystal on top of the glass.
The glass captures the mold's striped texture, thereby changing the original shape of the crystal in an illusory way. The timing of removal from the mold was too early, and the glass had not cooled to a fixed state, so an arc was formed.
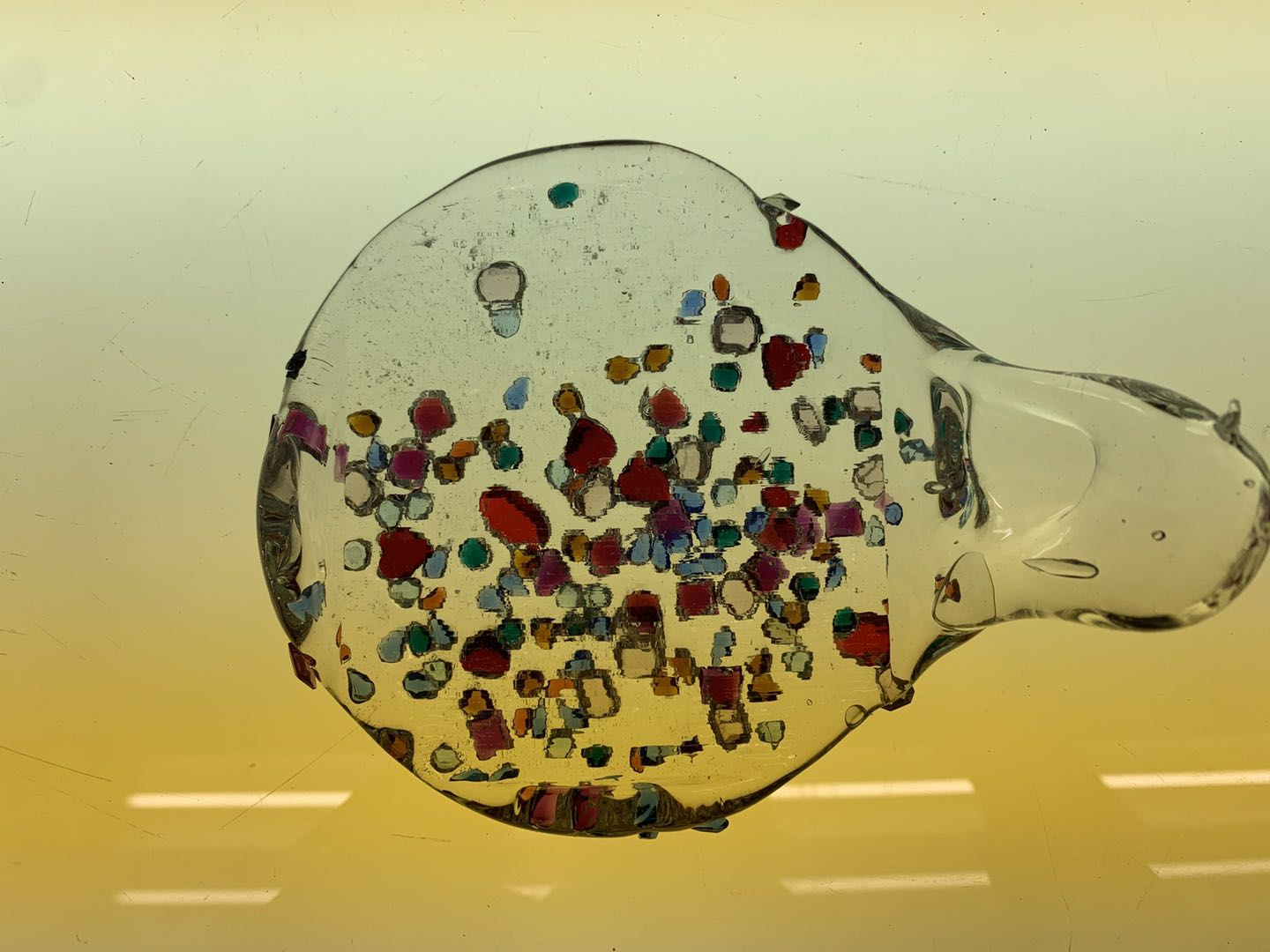





Attempt NO.3:
Hoped that the shape of the mold can be used to create a structure for the cooled glass.
Poured the molten glass on the top of the cylinder shape mold, then sprinkled the crystal on top of the glass.
Because there is not enough glass poured, there is not enough glass flowing down to form the structure I expect. But unexpectedly, the crystal sprinkled on the surface of the glass formed some small pits, which reminded me of the effect of upholstery furniture with buttons for soft decoration. It may be because the crystal was placed in time. Very inspiring.






Attempt NO.4:
Some crystals shattered in previous attempts, presumably because the indoor temperature crystal touched the molten glass, and the temperature difference was too extensive, so causing some large crystal to break. So this time, before the experiment, I preheated the crystal to over 1000 degrees Celsius, hoping that the crystal would not break.
Placed the crystal on top of the cylinder mold, then pour the molten glass over it.
Most of the crystals were not broken. Only a larger one was broken. The speculation may be correct, but the new assumption is that the larger the crystal, the easier it is to break.



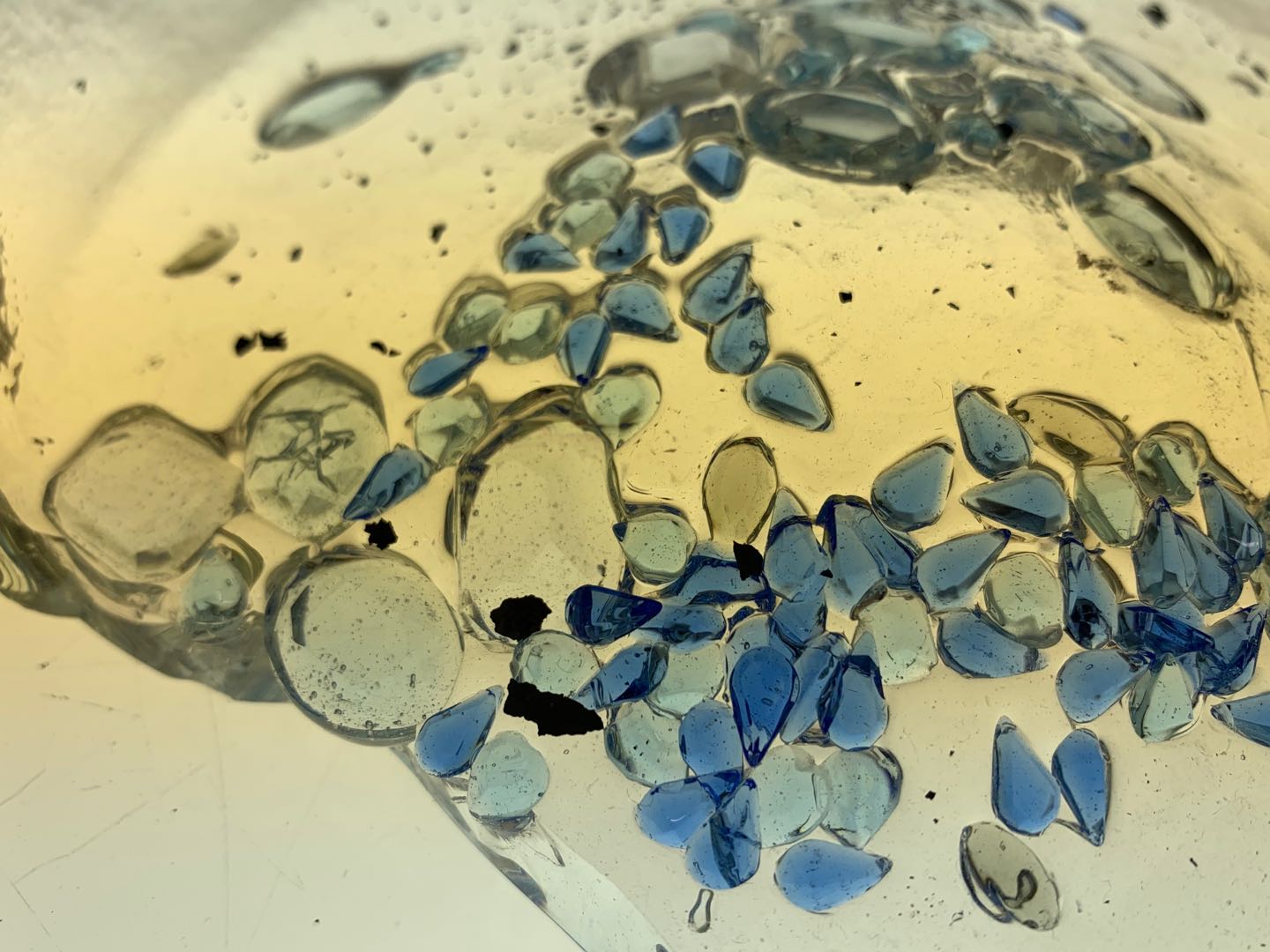
Attempt NO.5:
Hoped that I can give the glass a curvature through the steel mold that is made by myself.
Sprinkled the crystal on the board in advance. Poured the molten glass onto the slab to create a glass patty and then placed it on my mold before it cooled down.






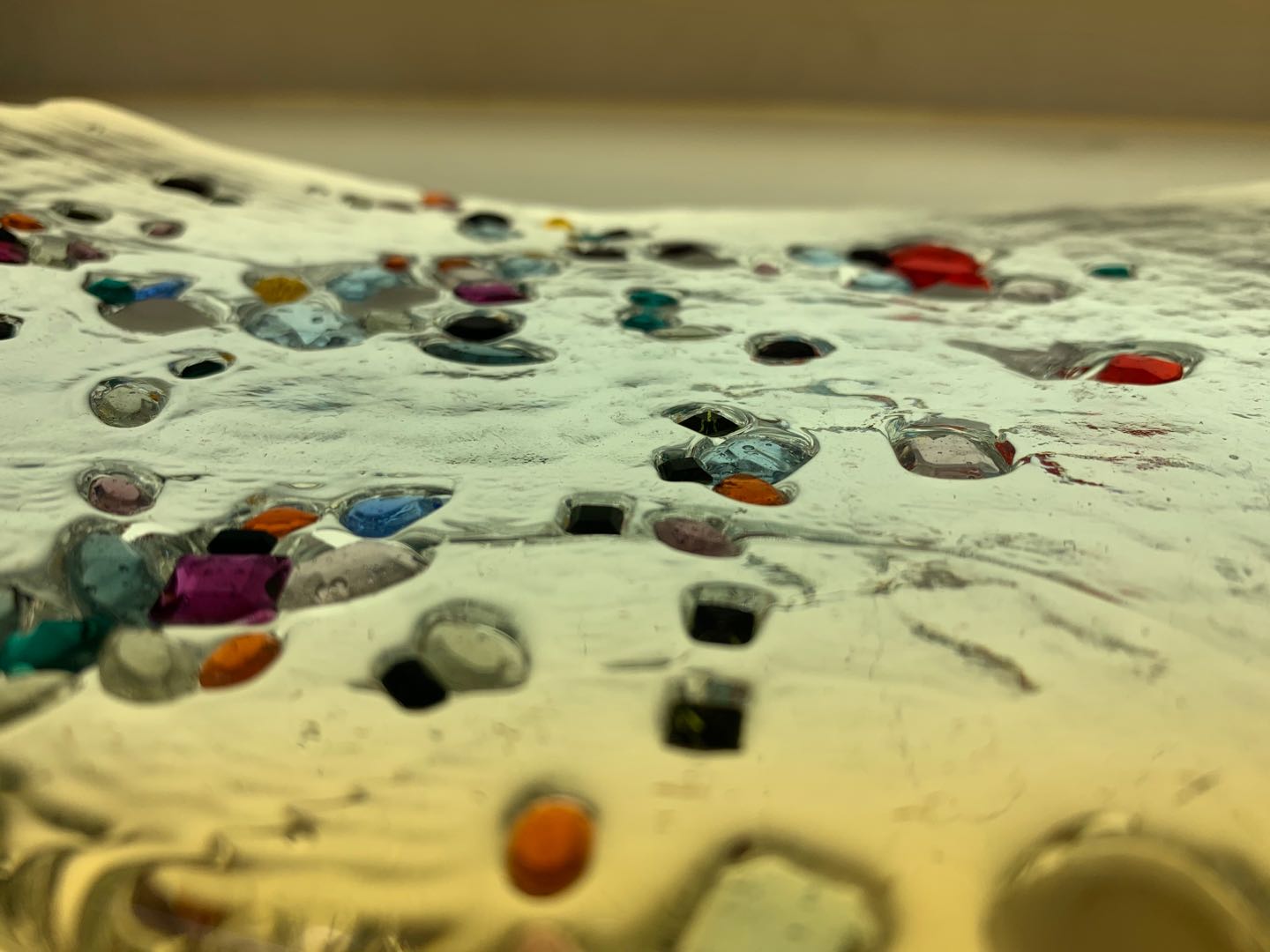

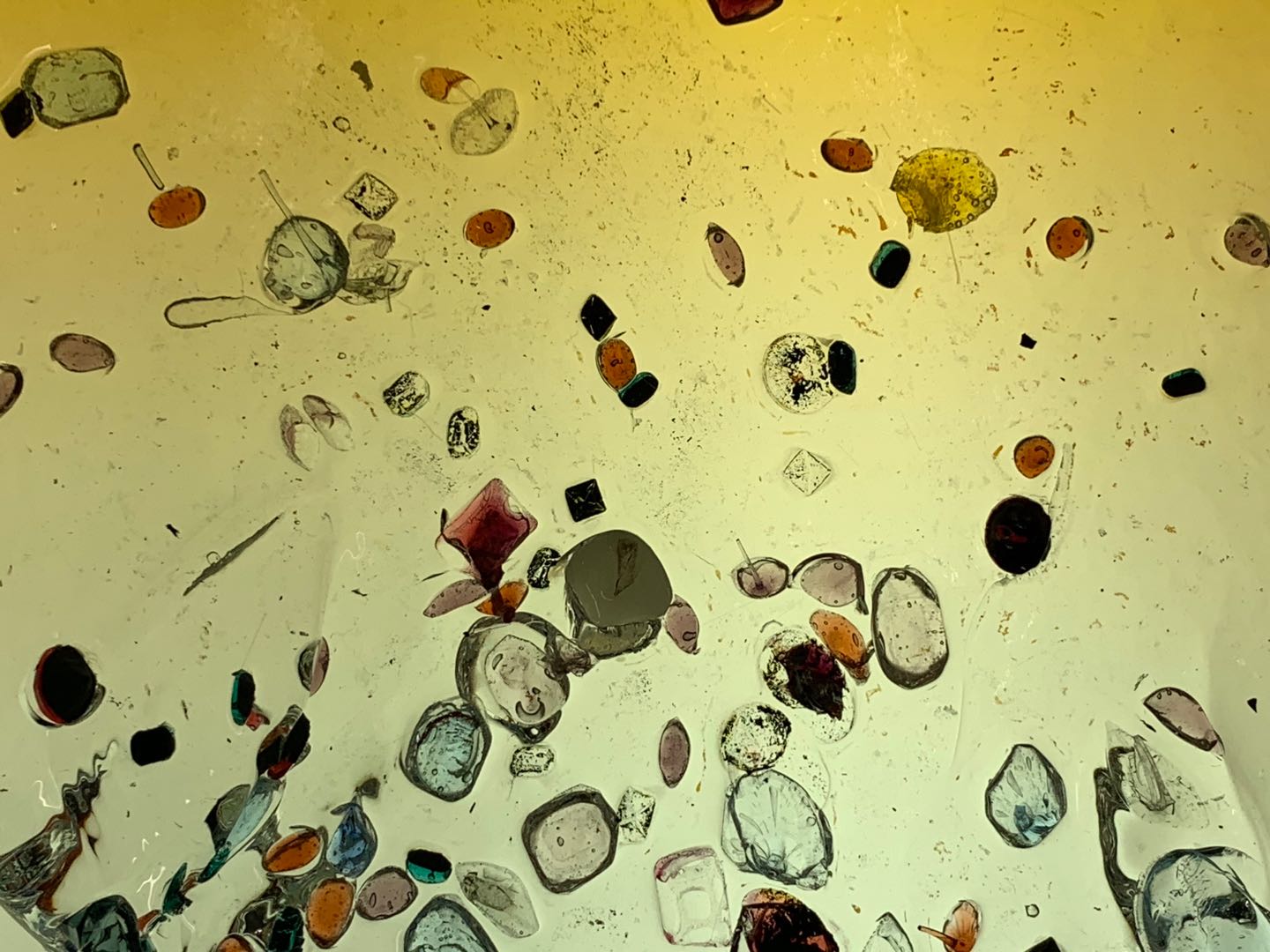
Attempt NO.6:
Hoped that I can give the glass a dome shape through the steel mold I made by myself.
Sprinkled the crystal on the board in advance. Poured the molten glass onto the slab to create a glass patty and then placed it on my mold before it cooled down.





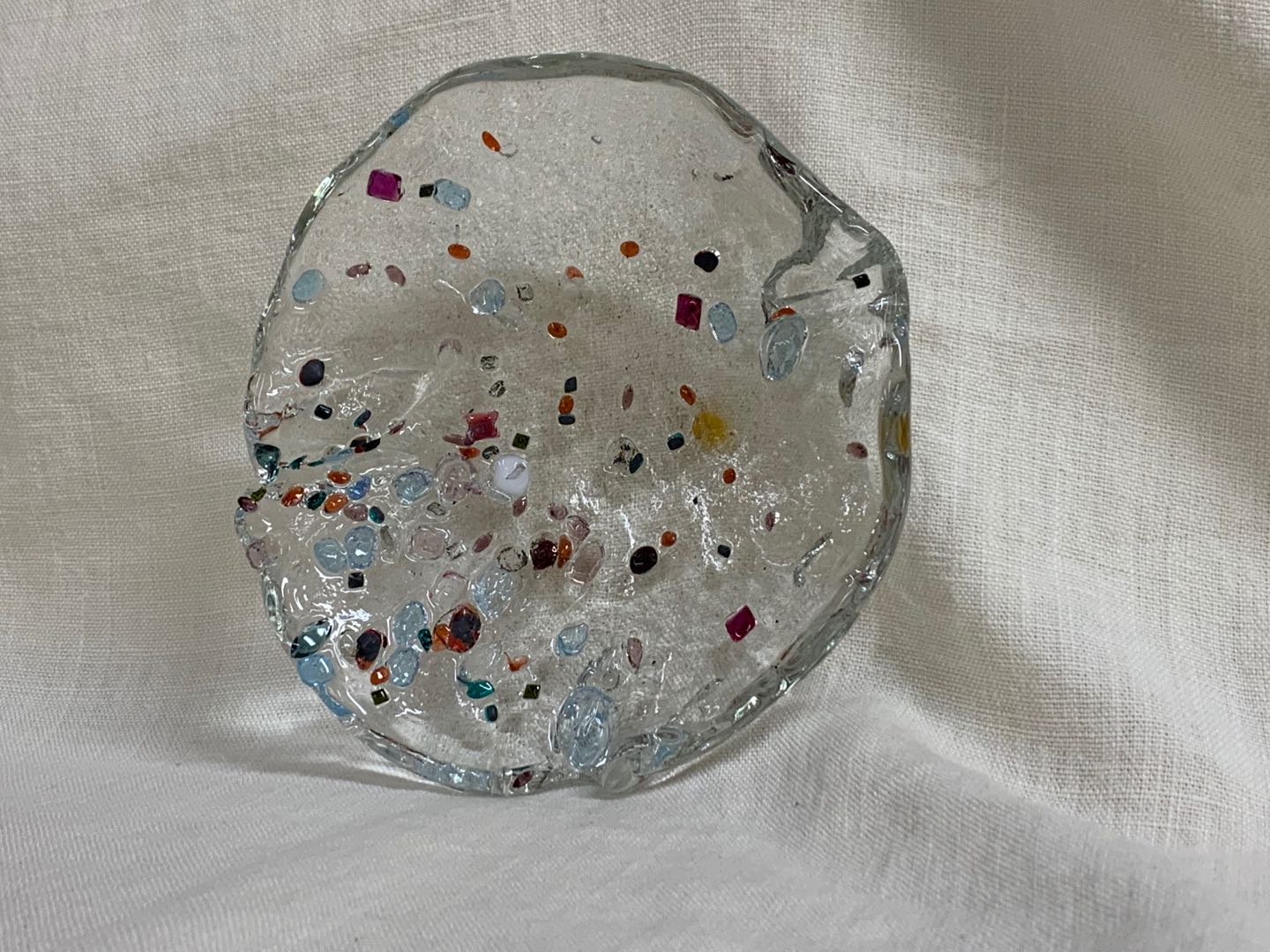

Attempt NO.7:
Found a huge cylinder shape mold in hot shop, I lay it down horizontally. Then poured the glass over it after I placed the crystal on the mold.







